Contents
Coordinated Ca2+ influx cascades for quantitative control of plant immunity
Coordinated Ca2+ influx cascades for quantitative control of plant immunity
Written by Dmitry Lapin, Junli Wang, and Jane E. Parker, August 2025
Original research article – Wang et al., 2025 (PNAS)
The plant immune system protects individual cells and tissues from invasion by other organisms. However, strong activation of defenses can have adverse effects on the normal host physiology, growth, and development. Beneficial microorganisms could be affected too. Therefore, it is essential to have checks on the immune response. A recent study led by a postdoctoral researcher Junli Wang in Jane Parker‘s laboratory at the Max Planck Institute for Plant Breeding Research in Germany (Cologne) identifies a new control point in plant immunity at the level of the Ca2+ flux into cells, leading to disease resistance.
Why is this study important?
In plants, the immune system can be activated through cell surface-localized and intracellular immune receptors. Perception of invaders by both receptor types leads to the influx of Ca2+ and its distribution within the cell. How this step occurs and what happens next remain big unresolved questions in plant immunology. Answering these questions will help understand the fine-regulation of plant defense and its effects on physiology.
Some background about TIR-domain signaling in plants
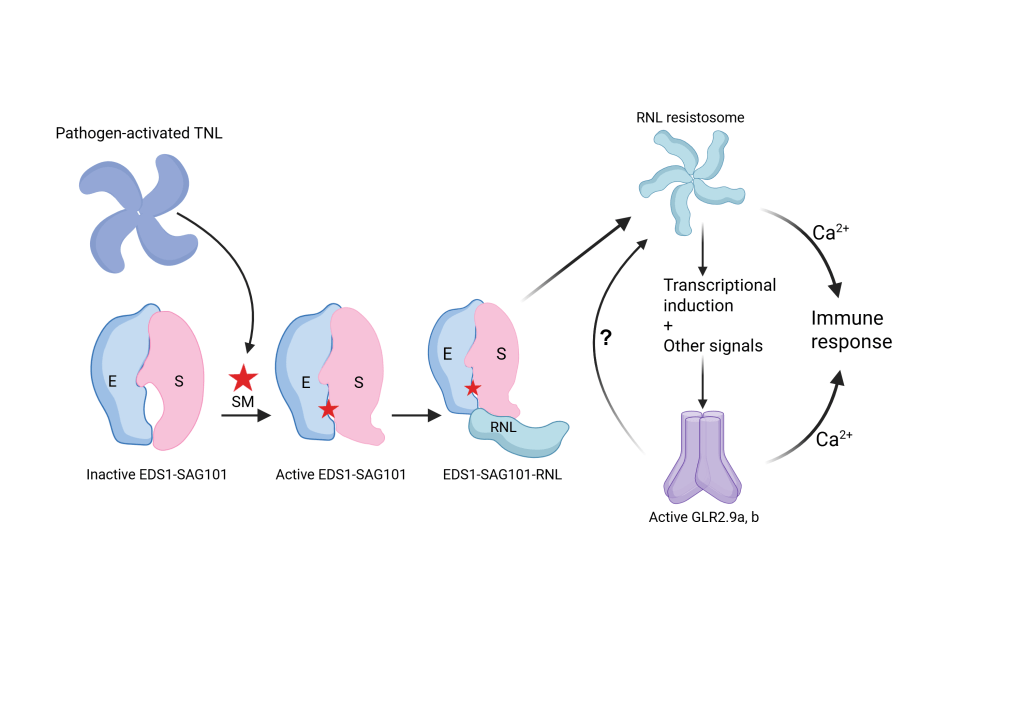
To help appreciate the importance of the study by Wang et al, we need to introduce signaling by the Toll/Interleukin-like Receptor (TIR) domain in plants. TIR signaling in plants is complex. Pathogens can activate TIR intracellular receptors, which leads to the formation of an enzyme from the receptors’ TIR domains. (Yes, an immune receptor can form a pathogen-inducible enzyme). This enzyme produces a range of small molecules from NAD+ and ATP. Particular small molecule catalytic products are then recognized by dimers of plant-specific proteins from the small enhanced disease susceptibility 1 (EDS1) family. These dimers then stimulate the assembly of a Ca2+ permeable channel from a specialized group of intracellular immune receptor-like proteins called RNLs.
Key findings of the study
Thanks to the study of Wang et al, we know some of what happens next. It turns out that the RNL-mediated Ca2+ influx promotes gene expression of members of another group of Ca2+ influx proteins, the glutamate-like receptors, GLRs, that are needed for fully effective immunity. Thus, TIR immune receptor signaling sets off a coordinated cascade of Ca2+ influx requiring two different groups of ion channel proteins.
We see that a strong immune response does not simply happen once a pathogen-detecting immune receptor is activated. Plants have evolved a sophisticated system of checkpoints to both stimulate defense and prevent it from over shooting in an uncontrolled manner. This tight control happens, among other processes, at the level of the Ca2+ influx regulation. For TIR-dependent immunity, we knew the regulated assembly of non-canonical functional RNL ion channel (see section ‘Further reading’). Now it is clear that transcriptional control of Ca2+ influx regulators is a next control point.
Another insight from the study is that immune signaling from cell surface and intracellular immune receptors does not necessarily follow the same path. These differences influence whether the plant cell can mount a strong immune response and even undergo cell death. Investigation of what determines the gene expression control of the GLRs can provide critical insights into the quantitative boosting of immune outputs.
A bit more background and details
Two glutamate receptor-like channels (GLRs) similar to animal ionotropic glutamate receptors (iGluRs) are further induced once a plant TIR-domain intracellular immune receptor is triggered in the model plant Nicotiana benthamiana. These plant GLRs contribute to Ca2+ influx, pathogen growth arrest, and host cell death. Earlier studies already established contributions of several types of Ca2+ channels in cell-surface and intracellular immune receptor signaling, such as Cyclic Nucleotide-Gated Channels, CNGCs. Also, cryoEM-guided functional analyses found that intracellular immune receptors with a coil-coiled signaling domain can assemble into Ca2+-permeable pores. The study by Wang et al shows that there is a coordinated Ca2+ influx dependent on ‘non-canonical’ ion channels during an early stage of a TIR-dependent immune response, which then promote canonical GLRs to confer strong disease resistance.
Final thoughts
Considering the number of immune regulators and their intricate connections, it seems unlikely that linear thinking will be sufficient to predict the outputs of immune system activation. Perhaps explicit modelling of these processes and closer collaboration between plant immunologists and mathematicians will help provide some of the answers.
Further reading
Control of TIR intracellular receptor signaling
Li, J., Chen, S., Yu, B. et al. TIR immune signalling is blocked by phosphorylation to maintain plant growth. Nat. Plants (2025). https://doi.org/10.1038/s41477-025-02012-x
Lapin, D., Johanndrees, O., Wu, Z., Li, X., Parker, JE. Molecular innovations in plant TIR-based immunity signaling. The Plant Cell (2022). https://doi.org/10.1093/plcell/koac035
Wu, Z. TIR innovations in plant immunity. New Phytologist (2025) https://doi.org/10.1111/nph.70314
Ca2+ influx control in plant immunity
Kim, N.H., Jacob, P., Dangl JL. Con-Ca2+-tenating plant immune responses via calcium-permeable cation channels. New Phytologist (2022) https://doi.org/10.1111/nph.18044
Modelling and immunity
Liu, X., Igarashi, D., Hillmer, RA., et al. Decomposition of dynamic transcriptomic responses during effector-triggered immunity reveals conserved responses in two distinct plant cell populations. Plant Communications (2024). https://doi.org/10.1016/j.xplc.2024.100882
Feedback control of the RNL assembly and function
Huang, S., Wang, J., Song, R. et al. Balanced plant helper NLR activation by a modified host protein complex. Nature (2025). https://doi.org/10.1038/s41586-024-08521-7
Xiao, Y., Wu, X., Wang, Z. et al. Activation and inhibition mechanisms of a plant helper NLR. Nature (2025). https://doi.org/10.1038/s41586-024-08517-3
Feehan, JM., Wang, J., Sun, X. et al. Oligomerization of a plant helper NLR requires cell-surface and intracellular immune receptor activation. PNAS (2023). https://doi.org/10.1073/pnas.2210406120